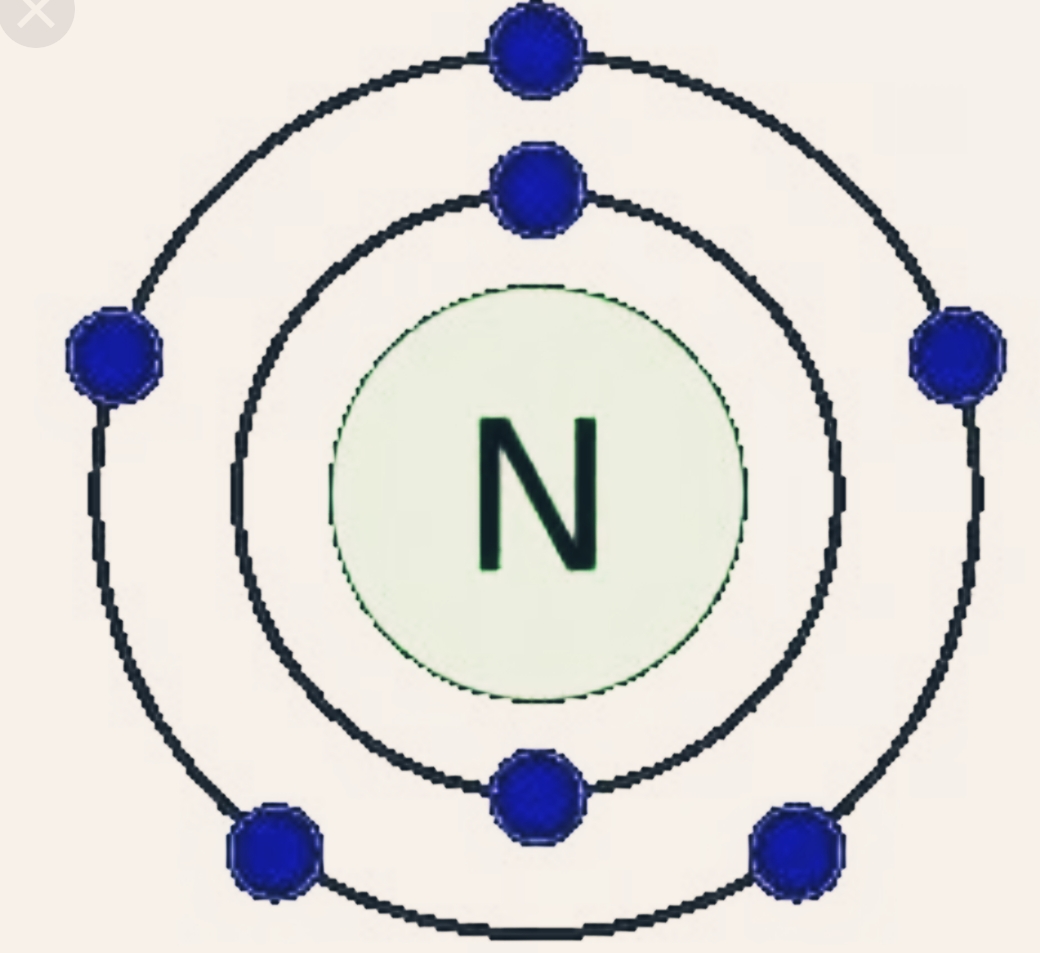
Nitrogen is a vital element that plays a significant role in various biological and chemical processes. With an atomic number of 7, this non-metal has attracted attention in both academic and industrial contexts. One of the key aspects of nitrogen chemistry lies in its valence electrons, which are crucial for bonding and reactivity. Understanding nitrogen valence electrons can provide insights into its behavior in compounds and interactions with other elements.
The valence electrons of nitrogen not only dictate its chemical properties but also determine how it interacts with other elements in forming molecules. This understanding is fundamental for students, researchers, and industry professionals working with nitrogen-rich compounds, such as fertilizers and explosives. By exploring nitrogen's valence electrons, we can unlock the secrets of its diverse applications in science and industry.
In this article, we will delve into the concept of nitrogen valence electrons, addressing essential questions and providing a comprehensive overview of their significance. From understanding what valence electrons are to exploring their role in nitrogen's chemical bonding, this article aims to enhance your knowledge and appreciation of this essential element.
What Are Valence Electrons?
Valence electrons are the outermost electrons in an atom that can participate in chemical bonding. They are crucial in determining how an atom interacts with others to form compounds. The number of valence electrons in an element can significantly influence its chemical properties and reactivity.
How Many Valence Electrons Does Nitrogen Have?
Nitrogen has five valence electrons. This is because it is located in group 15 of the periodic table, where elements have five electrons in their outermost shell. The arrangement of these electrons enables nitrogen to form a variety of compounds by sharing or transferring electrons with other elements.
Why Are Nitrogen Valence Electrons Important?
The nitrogen valence electrons play a pivotal role in chemical bonding. Their arrangement allows nitrogen to form stable covalent bonds with other atoms, leading to the creation of diverse molecules. For instance, nitrogen can form single, double, or even triple bonds, depending on the number of electrons shared with other elements. This versatility makes nitrogen a crucial component in organic and inorganic chemistry.
How Do Nitrogen Valence Electrons Affect Its Compounds?
The presence of five valence electrons allows nitrogen to form various compounds, including ammonia (NH₃), nitrogen dioxide (NO₂), and urea (CO(NH₂)₂). The ability to form multiple bonds with other elements results in a diverse range of chemical behaviors.
- Ammonia (NH₃): A compound formed by one nitrogen atom and three hydrogen atoms, showcasing nitrogen's ability to form single bonds.
- Nitrogen Dioxide (NO₂): A molecule formed by one nitrogen atom and two oxygen atoms, illustrating the potential for double bonds.
- Urea (CO(NH₂)₂): A compound that highlights nitrogen's versatility by forming covalent bonds with carbon and hydrogen.
What Role Do Nitrogen Valence Electrons Play in Biological Systems?
Nitrogen is an essential element for life, primarily as a component of amino acids, proteins, and nucleic acids. The nitrogen valence electrons enable the formation of crucial biological molecules that are fundamental to cellular processes.
How Do Nitrogen Valence Electrons Influence Fertilizers?
In agriculture, nitrogen-based fertilizers leverage the properties of nitrogen valence electrons to enhance plant growth. By providing plants with readily available nitrogen, these fertilizers can significantly improve crop yields. Understanding the chemistry behind nitrogen valence electrons helps in developing more efficient and environmentally friendly fertilizers.
Can Nitrogen Valence Electrons Form Bonds with Other Elements?
Yes, nitrogen valence electrons can form bonds with various elements. For example, in ammonia, nitrogen forms three single bonds with hydrogen atoms, while in nitrogen dioxide, it forms one double bond with one oxygen atom and a single bond with another.
How Does the Electron Configuration of Nitrogen Affect Its Reactivity?
The electron configuration of nitrogen is 1s² 2s² 2p³. This configuration shows that nitrogen has two electrons in its first shell and five in its second shell. The presence of three unpaired electrons in the 2p subshell allows nitrogen to easily form bonds with other elements, contributing to its reactivity.
What Are the Applications of Understanding Nitrogen Valence Electrons?
Comprehending nitrogen valence electrons is essential for various applications, including:
- Chemistry Research: Understanding nitrogen's behavior aids in developing new compounds and materials.
- Agriculture: Knowledge of nitrogen’s role in plant growth helps optimize fertilizer use.
- Environmental Science: Insights into nitrogen compounds can enhance pollution control strategies.
In conclusion, nitrogen valence electrons serve as a cornerstone in understanding the chemical and biological significance of nitrogen. Their unique properties allow nitrogen to participate in numerous reactions, making it an essential element in various fields, including agriculture, biology, and chemistry. By exploring the intricacies of nitrogen valence electrons, we can appreciate the pivotal role this element plays in sustaining life and advancing scientific knowledge.
You Might Also Like
Unraveling The Enigmatic Love Story Of Diana And DodiUnveiling The Mystique Of Super Saiyan God
Discovering The Flavorful World Of Chorizo And Eggs
Understanding National Credit Systems: A Comprehensive Overview
Unraveling The Mystery: Are Centipedes Poisonous?
Article Recommendations
- Nayib Bukele Religion
- Sela Ward
- Connor Mccaffery
- Jakerman
- Andy Bassich
- 16th November Zodiac
- Rod Argent
- Eazy E Wife
- Is Ellen Still Married To Portia
- Jordin Sparks