The electron configuration of an element is a crucial aspect of its chemical behavior and properties. When it comes to carbon, denoted by the symbol 'C', understanding its electron configuration is fundamental to grasping the building blocks of life. Carbon plays a central role in organic chemistry and is the foundation of many biological molecules. This article delves into the intricacies of the C electron configuration, providing insights into how this element interacts with others and contributes to the complexity of life on Earth.
In the world of chemistry, the arrangement of electrons in an atom determines how that atom will bond and react with others. For carbon, with its atomic number of 6, the C electron configuration is particularly interesting. It has four valence electrons in its outer shell, which allows it to form a wide variety of compounds, including the essential macromolecules like proteins, lipids, carbohydrates, and nucleic acids. Thus, understanding the C electron configuration not only enhances our knowledge of carbon itself but also sheds light on the intricate web of life that relies on this versatile element.
Throughout this article, we will explore various aspects of the C electron configuration, including its significance in chemical bonding, how it influences carbon's properties, and its role in the formation of complex molecules. Whether you are a student, a professional chemist, or simply curious about the nature of carbon, this comprehensive guide will help you appreciate the importance of the C electron configuration in both chemistry and biology.
What is the C Electron Configuration?
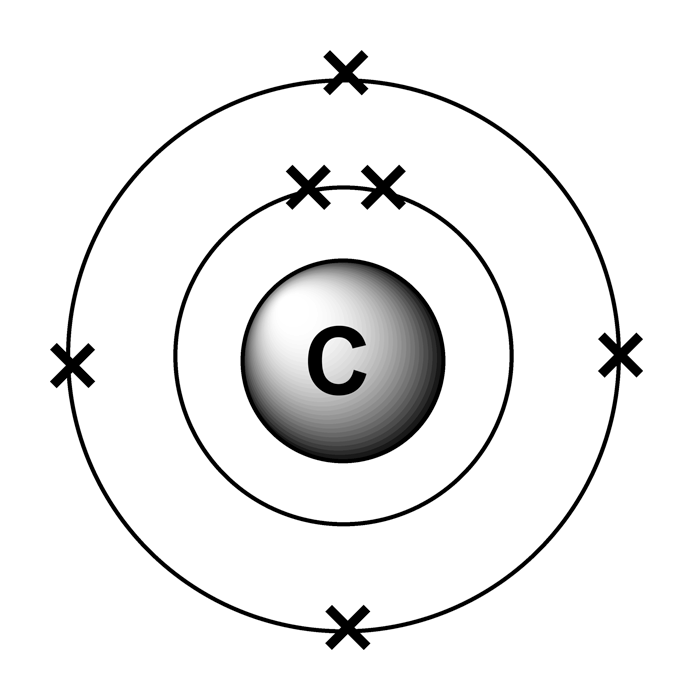
The C electron configuration refers to the distribution of electrons in the atomic orbitals of a carbon atom. For carbon, the electron configuration can be expressed as:
- 1s² 2s² 2p²
This notation indicates that carbon has two electrons in its first shell (1s) and four electrons in its second shell (2s and 2p). Understanding this configuration is vital for predicting how carbon will interact with other elements and form bonds.
How Does the C Electron Configuration Affect Chemical Bonding?
The C electron configuration significantly influences how carbon atoms bond with other atoms. With four valence electrons in its outer shell, carbon has the ability to form covalent bonds with a variety of elements, including hydrogen, oxygen, nitrogen, and other carbon atoms. This tetravalency is essential for creating the diverse range of organic compounds found in nature.
What Are the Types of Bonds Formed by Carbon?
Carbon can form several types of bonds, including:
- Single Bonds: Involves sharing one pair of electrons (e.g., CH₄ - methane).
- Double Bonds: Involves sharing two pairs of electrons (e.g., C₂H₄ - ethylene).
- Triple Bonds: Involves sharing three pairs of electrons (e.g., C₂H₂ - acetylene).
This versatility in bonding is what allows carbon to serve as a backbone for complex molecular structures, including long-chain hydrocarbons and intricate biological macromolecules.
Why is the C Electron Configuration Important in Organic Chemistry?
The C electron configuration is fundamental to the study of organic chemistry because it explains why carbon is often referred to as the "building block of life." The ability of carbon to form stable bonds with itself and other elements results in a vast array of organic compounds, which are essential for life's processes.
How Does Carbon's Tetravalency Contribute to Molecular Diversity?
Carbon's tetravalency allows it to form chains, branches, and rings, leading to an immense diversity of molecular structures. This molecular diversity is crucial for the development of various functional groups, which impart different chemical properties to organic compounds. Some examples include:
- Alcohols: Contain hydroxyl (-OH) groups.
- Amines: Contain amino (-NH₂) groups.
- Carboxylic Acids: Contain carboxyl (-COOH) groups.
This functional group diversity is what enables carbon-based molecules to perform a wide range of biological functions, from energy storage to catalysis.
What Role Does the C Electron Configuration Play in Biochemical Reactions?
The C electron configuration's influence extends to biochemical processes as well. Enzymes, which are proteins that catalyze biochemical reactions, often rely on carbon-based substrates to facilitate reactions. The specific arrangement of electrons in carbon-containing molecules determines their reactivity and interaction with enzymes, ultimately influencing metabolic pathways and cellular functions.
What Are the Implications of C Electron Configuration in Environmental Science?
The C electron configuration is not only relevant to chemistry and biology but also has significant implications in environmental science. The ability of carbon to form stable bonds allows it to exist in various forms, including carbon dioxide (CO₂), methane (CH₄), and organic matter. Understanding these forms is critical for studying processes like photosynthesis, respiration, and the carbon cycle.
How Does Carbon's Electron Configuration Relate to Climate Change?
The C electron configuration helps explain the behavior of carbon in the atmosphere and its contribution to climate change. Carbon dioxide, a byproduct of burning fossil fuels, traps heat in the atmosphere, leading to global warming. By comprehensively understanding carbon's behavior and interactions, scientists can develop strategies to mitigate climate change impacts.
What Are the Future Directions for Research on C Electron Configuration?
As scientists continue to explore the complexities of carbon and its electron configuration, future research may focus on:
- Developing new carbon-based materials with unique properties.
- Exploring carbon's role in alternative energy sources.
- Investigating carbon capture and storage technologies.
These advancements could have profound implications for sustainable development and environmental conservation.
In conclusion, the C electron configuration is a foundational concept in understanding the behavior of carbon, a vital element in chemistry, biology, and environmental science. Its significance extends far beyond the classroom, influencing critical areas of research and application that impact our world today. By unlocking the secrets of carbon's electron configuration, we can better appreciate the complexity of life and the importance of this remarkable element.
You Might Also Like
Mastering Game Development With Unity Tutorials From GeeksforGeeksUnderstanding The 120 Volt Outlet: A Comprehensive Guide
Unleashing The Power Of The AH 1Z Viper: A Modern Marvel In Aerial Warfare
Unveiling The Insights: Understanding Women Through Reddit's Ask Women Community
Mastering The Dispenser Recipe In Minecraft: Your Ultimate Guide
Article Recommendations
- Tyler Perry Spouse
- Joe Metheny
- Elijah Hewson Height
- P Diddy Gay
- Jessica Capshaw Husband
- Prince Harry Children 2024
- Morgan Fille
- Lee Hyun Woo Relationships
- Mychart Mainline
- Donald Trump Police Immunity